The Making of Life
Grappling With the Emergence of Life on Earth Helps Researchers Understand How to Search for It Elsewhere
Michael L. Wong is a research associate in the Univeristy of Washington’s Astrobiology program.
ARE WE ALONE?
This question looms larger with every passing year of envelope-pushing space exploration. For the first time in human history, we have the potential to find unambiguous signs of extraterrestrial life.
Liquid water is the most fundamental requirement for life as we know it. The physical and chemical properties of H2O control the molecular processes that underpin how life works. Water molecules dissolve ions and enable organic reactions that drive the essential functions of biology. Wherever we find liquid water on Earth, we find life. It’s no wonder that NASA adopted “follow the water” as the theme for its Mars exploration program.
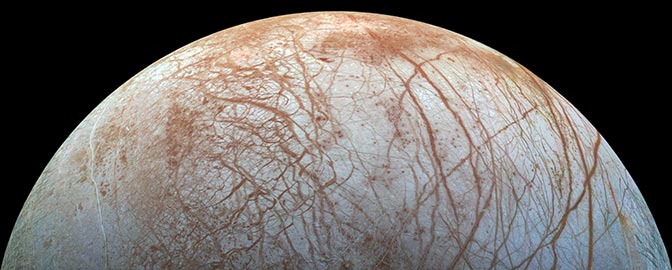
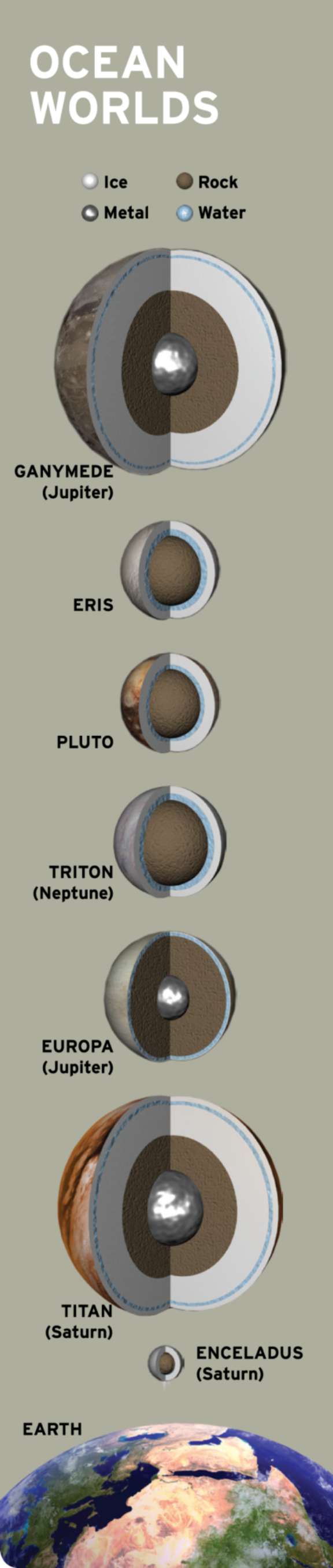
It turns out that water is everywhere in the solar system. A stable lake might hide beneath Mars’ south polar cap. Jupiter’s moon Europa houses perhaps twice as much liquid water as Earth. Tiny Enceladus squirts free samples of its ice-covered ocean into Saturn’s orbit. Titan has two kinds of fluids: a global layer of liquid water sloshes deep beneath a thick ice crust and the hydrocarbon seas on its surface. Add to this list the theoretical subsurface oceans of far-flung Pluto and Eris, and it seems that almost every world is staking its claim to habitability.
We now realize that about as many extrasolar planets exist as there are stars. Given the 100 billion stars in our galaxy and the trillions of galaxies in the observable universe, our cosmos should contain countless water-rich habitats.
But are they teeming with life?
Habitable vs. Inhabited
We used to believe that life spontaneously appeared wherever the conditions were right. In 1861, French scientist Louis Pasteur performed an experiment that refuted this concept of spontaneous generation, showing that life would only arise when a habitable but sterile environment was seeded by life from elsewhere. Life can only arise from life, Pasteur concluded.
In the present age of interplanetary exploration, Pasteur’s experiment serves as an important reminder that “habitable” is not synonymous with “inhabited.” Yet, if life is common across the cosmos, then abiogenesis—a synonym for spontaneous generation that doesn’t bear the latter’s historical baggage—must happen often enough to initiate life on worlds separated by vast tracts of sterile space.
The fact that you are reading The Planetary Report is proof that abiogenesis happened at least once in the universe’s history. However, until we find other such occurrences, we are forced to base our entire understanding of life on a single sample: us. Solving the mystery of how life emerged on Earth from nonliving processes is how astrobiologists hope to connect the concept of habitability to the reality of inhabitation.
CLUES FROM THE PAST
Almost every environment on Earth has been infected by—or at least affected by—biology, from microbes to humans. Life gave us an oxygen-rich atmosphere, introduced thousands of new minerals to Earth’s crust, broke rocks apart, held sediment together, sent the world into a global deep freeze, and is now steadily warming the climate.
Despite Earth’s incredible capacity to host life, there is no evidence that spontaneous generation has happened more than once. Just as Pasteur surmised, the life that exists today arose from life that came before it, which came from older life made by even older life, on and on and on. We know this because we can connect the dots between every living thing that we have discovered on a phylogenetic tree. Thanks to our ability to read the instructions written in DNA and RNA, we can compare genetic codes across every domain of life and draw a map of evolution stretching back to our last universal common ancestor, charmingly referred to as “LUCA.” The identity of LUCA has been lost to history, but through fossil evidence, we know that life has persisted on this planet in one form or another for roughly 4 billion years.
Thus, the very first life form on Earth emerged about one third the age of the universe ago. The conditions of the early Earth—which were nothing like what we experience now—might have been much more conducive to the emergence of life.
IT CAME FROM THE DEEP
You would die instantly if you were transported back in time by 4 billion years, asphyxiating in an oxygen-free environment and succumbing to high doses of ultraviolet radiation from the young Sun. Even if you circumvented those calamities, you’d eventually drown if you didn’t have the foresight to bring a boat because there were probably no landmasses on the infant Earth.
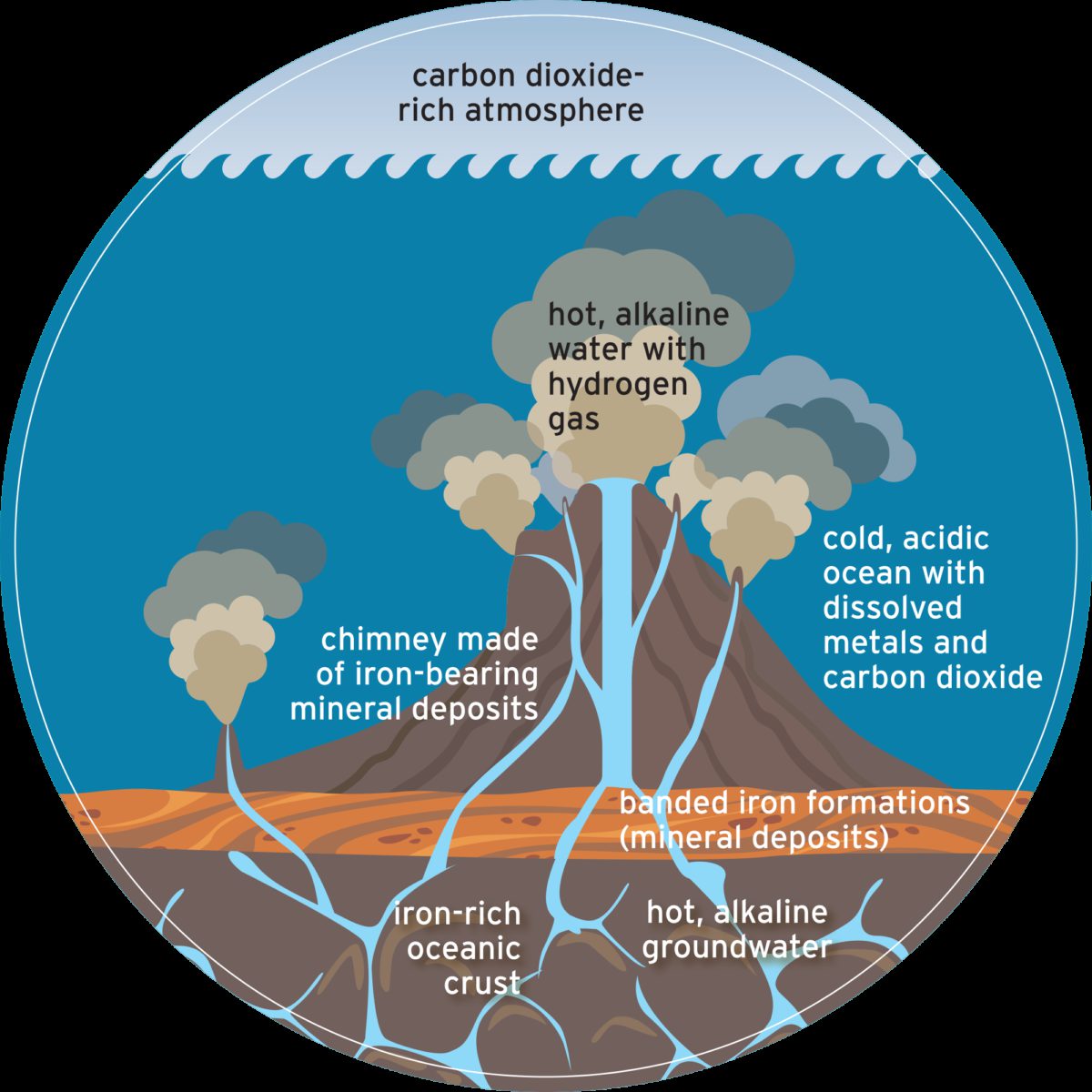
How could anything resembling life possibly originate at the surface of such a treacherous world? The short answer is: it probably didn’t. Instead, a growing body of scientific work has come to suggest that life emerged at hydrothermal vents at the bottom of the ocean.
Let’s say your time machine was also a submarine, one that could dive to the base of Earth’s early ocean. There, you would find spires resembling the chimneys of the Atlantic Ocean’s Lost City hydrothermal field. These massive structures, some more than 50 meters tall, were created by the precipitation of iron-bearing minerals when two very different kinds of water met.
The majority of Earth’s ancient ocean was acidic like a lightly carbonated soft drink, thanks to an overlying carbon dioxide–rich atmosphere. Just beneath the ocean floor, seawater and rock interacted in a process called serpentinization. This chemical reaction changed the water’s pH, rendering it alkaline (the opposite of acidic). It also heated the water and infused it with molecular hydrogen, a valuable chemical fuel for life.
When this alkaline groundwater seeped into the ocean, it found itself out of equilibrium with the surrounding colder, acidic, carbon dioxide–rich seawater. “Out of equilibrium” is just jargon for “unbalanced,” but its ramifications for life are enormous. An out-of-equilibrium situation is full of untapped energy—the potential to enact change and create complexity.
ELECTRONS AND PROTONS POWER LIFE
Consider how disequilibria power humans. We eat and breathe to gain energy, but what does that really mean? At its heart, it’s all about electrons. Our bodies take electrons from the electron-rich food that we eat and transfer them to the electron-greedy oxygen in the air that we breathe.
This electron transfer, mediated by a series of chemical reactions that happen inside of our cells’ mitochondria, releases useful energy. Proteins in our mitochondria use this energy to pump protons across an inner membrane, transforming what used to be an imbalance in electrons into an imbalance in protons. A protein called ATP synthase uses the potential energy stored in the imbalance of protons to create adenosine triphosphate (ATP) molecules. Actually, ATP exists as part of yet another imbalance: that between its wholesome self and its broken pieces, adenosine diphosphate and a lone phosphate. ATP is often thought of as the “energy currency of life,” and now the origin of that stored energy is clear: it’s the useful energy derived from a disequilibrium.


It’s not just our cells that harness these disequilibria to create ATP. Almost all the other living things on Earth do too, down to the most primitive single-celled organisms. It’s so universal, in fact, that these disequilibria might even be related to how life started.
A HYDROTHERMAL HATCHERY OF LIFE
Back in your time-traveling submarine 4 billion years in the past, you put a microscope up against a hydrothermal chimney. You find that this colossal tower is built like a high-rise apartment building with trillions of tiny mineral rooms, or vesicles, each roughly the size of a biological cell.
Inside these vesicles, H2 from serpentinization and CO2 from the surrounding seawater swirl in chemical disequilibrium. In this case, H2 is the fuel (electron donor) and carbon dioxide is the air (electron acceptor). There’s a second disequilibrium present between the alkaline vent fluid and the acidic seawater. The contrast in pH is a natural proton imbalance that resembles the proton imbalance created in modern-day cells.

We don’t know how life really began, but here’s a plausible scenario involving the untapped geochemical energy present in these ancient hydrothermal chimneys. Travel forward through time and you might see H2 and CO2 react with each other, aided by the catalytic metals in the vesicle’s walls. They would not only form organic molecules but release pent-up energy as well, and if some organic-mineral precursor to ATP synthase could use the natural proton gradient to bind phosphates together, an energy currency is within the realm of possibility. Incorporating nitrogen- and sulfur-bearing molecules dissolved in the surrounding water, these processes could lead to the first metabolic network: a web of reactions that reinforces itself, growing more stable and more complex with time.
Eventually, this network might lead to information-carrying and self-replicating molecules like RNA, allowing for greater adaptive abilities to changing conditions. It might also construct lipid membranes to replace the inflexible and immutable mineral walls and build ion pumps to regulate its own proton imbalance, thereby effecting the ability to escape these hydrothermal confines.
In the end, it would be a fully functional cell—a product of its geochemical past afloat in the formerly sterile abyss of this ancient sea, soon to encounter new places to thrive and evolve into new ways of being.

AN EVER-EVOLVING FIELD
For all of its attractive aspects, nobody knows whether this story represents primordial reality. As you read this, scientists are testing various aspects of the hydrothermal-vent hypothesis. Some are learning more about how chemical disequilibria create complex structures by making hydrothermal analogs in the lab. Others are conducting experiments on the catalytic properties of metal-bearing minerals. Still others are investigating how the temperature disparity between the vents’ hot interiors and cold exteriors could help concentrate the organic components of life. A few are even trying to come up with clever ideas for the structure of ATP synthase’s precursor and how proto-metabolic networks stored and carried information.
Some scientists are investigating completely different hypotheses for the origin of life. Many of these involve prebiotic soups of complex organic molecules. These organic medleys sunbathe on Earth’s surface until just the right combination of them find each other to form life. In the vast expanse of Earth’s primitive ocean, the chance encounters between potential molecular collaborators would be rare. So, most researchers suspect that the organic matchmaking must have occurred at tidal pools along the seashore or in freshwater hydrothermal pools, where periodic drying episodes promoted the concentration and polymerization (or “sticking together”) of life’s building blocks.
Origin-of-life research doesn’t lack in “far out” ideas either. One camp argues that life on Earth began as self-replicating clay minerals. Another group claims that nuclear-powered geysers—formed by the decay of radioactive uranium—enabled the prebiotic reactions that formed life.

Then there’s the notion that we’re all Martians, insisted upon by those who consider early Mars a likelier place to start life than early Earth. In this scenario, primitive Martian microbes hitched a ride deep inside an impact-ejected rock and seeded our planet in a process known as lithopanspermia.
If any of these ideas proves correct, what would that imply about our loneliness in the cosmos?
SEEKING OUR COSMIC NEIGHBORS
If life originated because of the physical and chemical disequilibria at hydrothermal vents, then countless wet, rocky planetoids should provide the basic requirements to start life. Hydrothermal systems can result from water meeting rock on any tectonically active world. Martian rocks examined by the Spirit rover bear the mineral byproducts of ancient hydrothermal systems, and Cassini identified the telltale signs of hydrothermal activity on the Saturnian moon Enceladus. Thus far, the hydrothermal-vent hypothesis is the only scheme that could plausibly lead to independent abiogeneses on both Earth-like planets and ice-covered ocean worlds.
However, if the emergence of life requires surface environments that undergo wet-dry cycles and are directly exposed to air and radiation, then Mars would be much more conducive to life than Europa or Enceladus are. In this case, the icy satellites of Jupiter and Saturn—as well as their analogs across the cosmos—would be habitable but sterile, barring the unlikely scenario that some rock ejected from an inhabited world like Earth seeded them.
If life can arise in a chemical soup without the aid of catalytic minerals or tectonic activity, then that raises the possibility of exotic life on the surface of Titan. This moon of Saturn produces complex organic molecules in its atmosphere that collect on its surface and in its hydrocarbon seas. Scientists look to Titan for potential analogs to the organic-rich soups that were present on Earth’s first landmasses.
At present, there is no consensus in the scientific community on the requirements for the origin of life. Perhaps none of our hypotheses are correct, or perhaps the answer is “all of them.” We just don’t know yet.
Finding life on any neighboring world would tell us which—if any—of our origin hypotheses are more likely than the others. Not finding life would also teach us that habitability alone is an insufficient condition for life. Now that we know that such a diverse array of astrobiological candidates exist in our own cosmic backyard, the question begs: is anyone out there?
Let’s Go Beyond The Horizon
Every success in space exploration is the result of the community of space enthusiasts, like you, who believe it is important. You can help usher in the next great era of space exploration with your gift today.
Donate TodayThe Planetary Report • December Solstice
Help advance space science and exploration! Become a member of The Planetary Society and you'll receive the full PDF and print versions of The Planetary Report.


 Explore Worlds
Explore Worlds Find Life
Find Life Defend Earth
Defend Earth