Emily Lakdawalla • Oct 15, 2013
DPS 2013: Confusing Curiosity SAM results
What did I learn about Curiosity at last week's Division for Planetary Sciences meeting? DPS is fundamentally an astronomy meeting, so it doesn't usually feature much surface Mars science. (Of the fall-season meetings, Mars geologists generally prefer the American Geophysical Union meeting in December or even the Geological Society of America meeting, later in October.) But there were a few talks, most of which concerned soil and atmsospheric chemistry. I can summarize their conclusions with one sentence: More data is needed.
Caroline Freissinet presented about SAM results on detections of carbon-containing compounds in both the Rocknest and Yellowknife Bay samples. (SAM is the fiendishly complex sample-cooking gas-sniffing Sample Analysis at Mars analytical laboratory instrument suite, which I explained in an earlier post.) Among SAM's major goals is to detect organic compounds on Mars. SAM has detected some, but it's not totally clear whether they represent Martian organics. To explain why is a little complicated, but here goes.
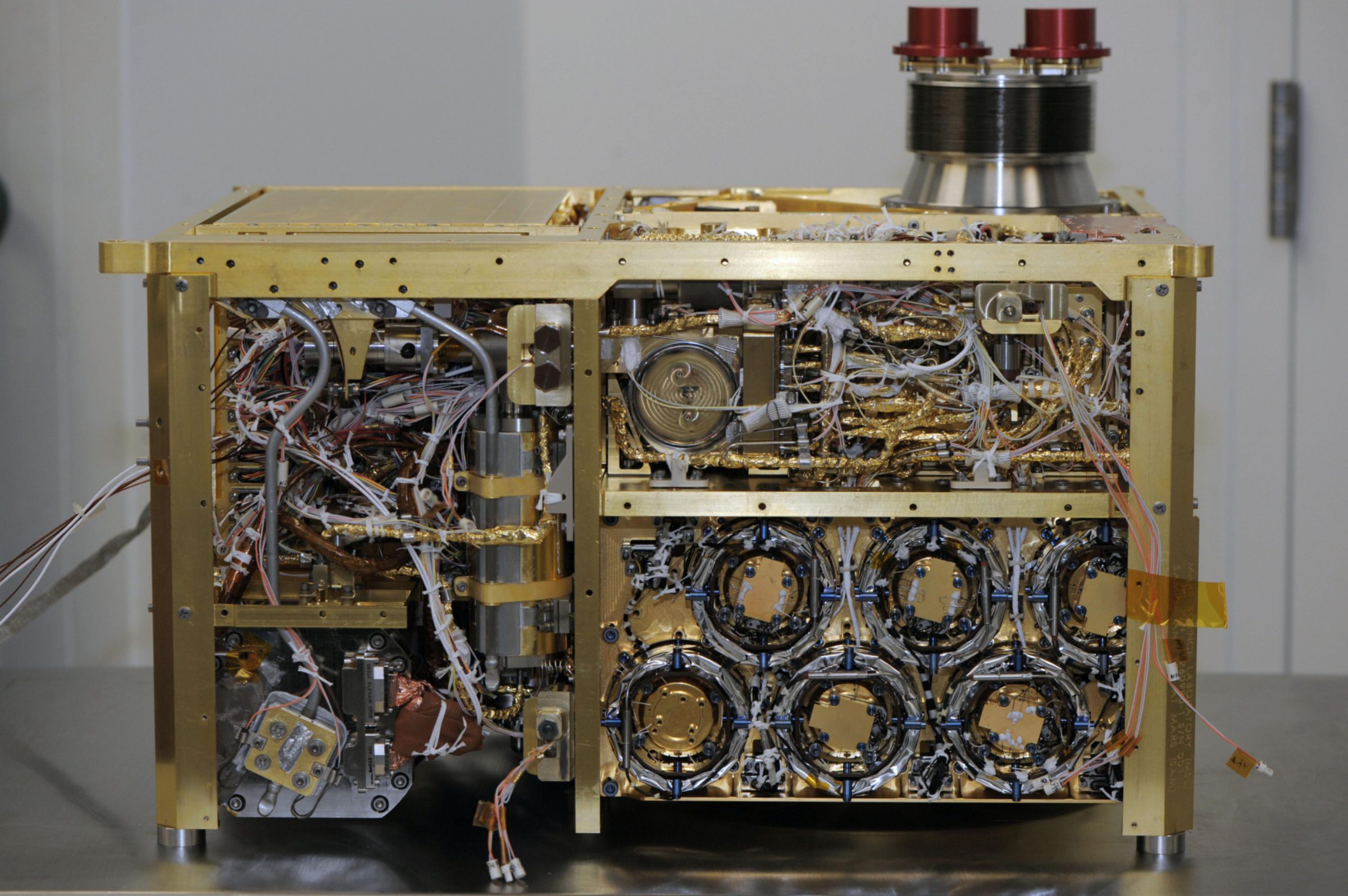
The first problem that SAM faces is that it contains a contaminant that could be confusing the results. SAM contains a few "wet chemistry" cells, pre-filled with a substance called N-Methyl-N-tert-butyldimethylsilyltrifluoroacetamide, a mouthful that is usually abbreviated to the not-much-better "MTBSTFA." This substance reacts with organic molecules to form new substances that are easier to turn into gases but harder to break down with heat. That makes the hopefully Martian organic compound more likely to survive the SAM oven's heating without combusting into simpler compounds, and also makes it more likely to go into a gaseous phase that will flow through SAM's gas chromatograph mass spectrometer so that SAM can figure out what organic compound it actually is.
The problem is, it seems that some of this MTBSTFA has leaked within SAM. MTBSTFA is an organic compound, so when SAM detects organic compounds, we can't know if they came from the Martian sample or if they are just contaminants from the leaked MTBSTFA. Which is a big pain.
Freissinet presented some work showing progress at correcting for the MTBSTFA contamination. They have developed a new testing routine. Before they dump a sample into a SAM cup, they first pump as much gas out of the system as they can. Then they preheat the cup to 150 degrees Celsius. Then they deliver three portions of sample to the cup, rather than just one. When they test just a single portion of sample without this pre-treatment routine, they detect the equivalent of about 32 nanomoles of gases that were derived from the MTBSTFA. When they test a triple portion of the sample using the pre-treatment routine, they detect only 9 nanomoles of MTBSTFA in the sample.
In all of the solid samples that they have tested so far, which includes sand from the Rocknest sand drift and drilled rock from Yellowknife Bay, they have detected carbon compounds that also have chlorine atoms attached to them. These include chloromethane (which is like methane, CH4, except there's a chlorine atom substituted for one hydrogen atom, so it's CH3Cl), as well as dichloromethane (CH2Cl2), trichloromethate (CHCl3) and tetrachloromethane (CCl4), plus chlorobenzene (which is a benzene ring, C6H6, except one of the hydrogens is replaced with a chlorine). There isn't any chlorine in the MTBSTFA, so the chlorine atoms come from Mars, very likely from perchlorate ion (ClO4-), a highly reactive ion that was also detected by Phoenix. The question is, does the carbon come from Mars, or does it come from Martian perchlorate reacting with MTBSTFA?
Freissinet compared samples analyzed with and without the pre-treatment routine. She showed that, in the Yellowknife Bay but not the Rocknest sample, the amount of chlorine-bearing hydrocarbons was different with and without pre-treatment. Specifically, when the test was repeated with the pre-treatment routine, there was less chloromethane and dichloromethane, the same amount of trichloromethane and tetrachloromethane, and an increase in the amount of chlorobenzene. It's the last one that gave her hope: she cautiously believes that the benzene rings bonded to chlorine atoms represent Martian carbon from Martian organic compounds that reacted upon heating in the presence of perchlorate ion to become chlorobenzene.
Freissinet closed with an interesting statement: whatever the origin of the carbon in these compounds, there is not a wide variety of carbon compounds present in the samples. She didn't elaborate on that point, but wherever you have naturally occurring carbonaceous material you usually have a lot of variety in the carbon-containing species present. So that's a little odd.
In the same session, Michael Wong presented some very confusing results on atmospheric isotopes measured by Curiosity, using SAM's Quadrupole Mass Spectrometer (QMS). QMS measured the proportions of nitrogen-14, nitrogen-15, and argon-40. Previous measurements of the nitrogen isotope ratios of gases trapped in Martian meteorites matched fine with previously reported results from Viking. In contrast, the Curiosity results do not match -- the argon/nitrogen ratio is quite different for Curiosity than it was for Viking, and it doesn't fit with the ratio measured in Martian meteorites. Wong said that the team has "looked into this extensively" and that they "find no reason to doubt the MSL data." They brought calibration gases with them, and have measured them, and the measurements match reality. "So we're scratching our heads right now," he said. He speculated on possible causes: has there been a change in the atmosphere since the time that the meteorites were ejected, or between Viking and the present? Comparisons to Viking are challenging, he said, because no one can find the original, raw Viking data (in particular, the background measurements).
If the ratio of argon to nitrogen is confusing, the ratio of argon isotopes is not, as Sushil Atreya stated later in a plenary talk. The argon isotope ratios (the proportion of argon-36 to argon-38) measured by SAM "gives the best evidence yet that Martian meteorites actually came from Mars" and a clear signal of the loss of Mars' atmosphere. The measurement of the argon isotope ratio by Viking was ambiguous -- it covered a wide range, including the present-day Earth value -- but SAM's value indicates that Mars has lost 50 to 95% of its primordial atmosphere, with "most people edging toward the higher end."
Scientists always say that more work is needed, Freissinet, Wong, and Atreya did no differently at DPS. But Curiosity is definitely a situation in which much more work is needed. There are no quick answers on this mission. And it's going to be quite a while until we get the data we need to answer the questions.
Atreya said that the motto of the Curiosity mission right now is "Mount Sharp or Bust." They have been driving, driving, driving, and have covered roughly 3 of the total 9 kilometers they have to traverse to get to the base of Mount Sharp. They are coming up on a spot identified as "Waypoint 2" on the long-distance traverse map, but according to the conversations I had with people at the meeting, it seems likely they may skip the stop and just keep driving. People told me that they were under a lot of pressure to get to Mount Sharp as fast as possible, and that means no dilly-dallying for science along the way at rocks that just don't show much evidence for interesting mineralogy from orbit. These are not the rocks we're looking for.
Just to make an editorial comment, I'm happy that the team is sharing their confusion with their peers. They could just not show up and not talk when they aren't sure of their conclusions yet. Or they could show their data and discuss the range of possible interpretations and explain why figuring out the answer is so hard. I'm glad we have the window into the challenging Curiosity mission that the latter approach allows us.
Let’s Go Beyond The Horizon
Every success in space exploration is the result of the community of space enthusiasts, like you, who believe it is important. You can help usher in the next great era of space exploration with your gift today.
Donate Today

 Explore Worlds
Explore Worlds Find Life
Find Life Defend Earth
Defend Earth

